Electric vehicles are critical to reducing transportation pollution and solving the climate crisis, but manufacturing them at the necessary scale will require significantly increasing production of the batteries that power them. How batteries are made, what they are made of, and whether they are reused or recycled affect the sustainability of this crucial component.
Even though batteries last many years, they eventually reach the end of their useful life for powering electric vehicles. Policies and incentives for recycling and reusing batteries, including strong health and labor standards, will further lessen the impacts of electric vehicles.
Electric Vehicle Batteries
This is a condensed, online version of the report. Access to all figures and full report are available through download of the PDF.
Battery electric vehicles (BEVs) are a key strategy for reducing air pollution and global warming emissions. They have zero tailpipe emissions, and even when powered by today’s sources of electricity, their life cycle global warming emissions are significantly lower than those for vehicles fueled with gasoline or diesel (O’Dea 2019; Reichmuth 2020). However, the increased adoption of BEVs raises important questions about the availability, recyclability, and sustainability of battery materials.
Scaling up BEV manufacturing will mean increasing the production and processing of several materials used in today’s lithium-ion batteries. Fortunately, strategies for recycling lithium-ion batteries offer the possibility of a sustainable, long-term supply of such materials, supporting the continued deployment of electric vehicles (EVs). However, implementing those strategies will require addressing a number of technical, economic, logistic, and regulatory barriers.
Battery Materials and Their Availability
Battery packs in EVs contain hundreds, even thousands, of individual lithium-ion batteries, typically referred to as cells and often similar in size to AA alkaline batteries. Cells consist of two electrodes: the anode (the negative terminal of a battery in use) and the cathode (the positive terminal). When the battery is operating (discharging), lithium ions move from the anode to the cathode through an electrolyte (often a liquid) and a plastic separator that prevents the anode and cathode from coming into contact and short circuiting the cell. Electrons flow around the separator from the anode to the cathode through the device powered by the battery.
To facilitate smooth charging and discharging, battery packs consist of multiple cells bundled into modules. Combining several modules with additional packaging and thermal management systems creates the finished battery pack used in EVs.
Of the materials used in lithium-ion battery cells, the US government deems many to be “critical” (Box 1) (DOI 2018). These elements are crucial to battery performance, yet their supply is at risk, whether due to material shortages or because supplies are concentrated or processed in a single country (Bauer et al. 2010).
Different types of lithium-ion batteries are distinguished by the metals that make up the cathode. The choice of materials affects important battery characteristics such as longevity, cost, and energy density (the amount of energy a certain size battery stores). The choice also affects other battery components, such as thermal and power management systems.
The cathode, which accounts for roughly one-quarter of the cost of a battery, combines lithium with nickel, manganese, cobalt, aluminum, or iron. Aluminum is also used as the cathode’s current collector and in packaging for the cell and module. The anode typically consists of graphite and a copper current collector.
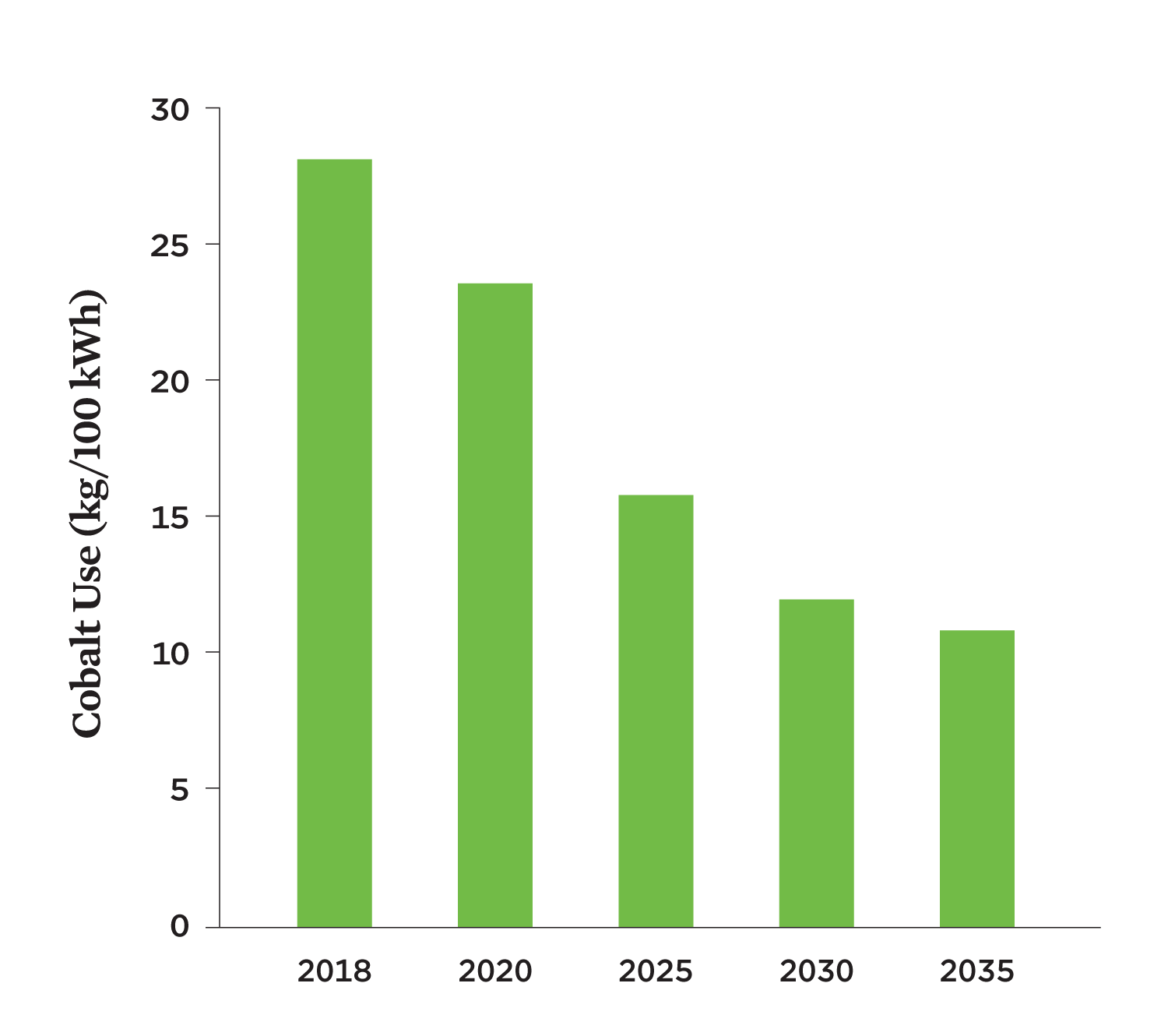
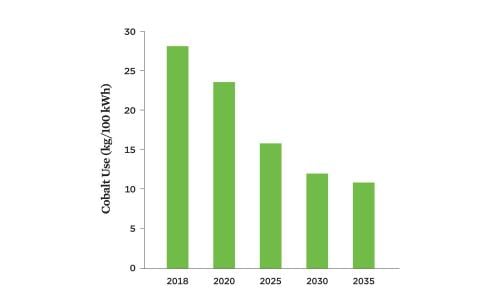
Early lithium-ion battery cathodes relied heavily on cobalt. Today’s batteries use less cobalt per kilowatt-hour (kWh) of energy capacity, although it is still commonly used because it contributes to a battery’s energy density and chemical stability. Both the high price of cobalt and negative impacts of mining it motivate efforts to reduce the amount of cobalt in batteries. In 2018, lithium-ion batteries averaged 28 kilograms of cobalt per 100 kWh across all battery end uses and chemistries. This amount is expected to decrease by 60 percent by 2035 (Figure 1, p. 3).
New and low-cobalt cathode chemistries can offer improved battery performance through higher energy densities. Battery cathodes using less cobalt include nickel-cobalt-aluminum oxide (“NCA”) and some nickel-manganese-cobalt oxide (“NMC”) compositions. In addition, major manufacturers of light- and heavy-duty BEVs widely use cobalt-free cathodes based on lithium iron phosphate (“LFP”).1
Still, even with significant reductions in the amount of cobalt or other critical materials, overall consumption will rise as more EVs are produced and the capacity of each vehicle’s battery increases. More than 60 gigawatt-hours (GWh)2 of lithium-ion battery capacity has been deployed in roughly 1 million BEVs in the United States since 2010. EVs sold in 2019 alone accounted for more than one-quarter of the total battery capacity deployed (16 GWh) (Ambrose et al. 2020). The significant role of BEVs in reducing emissions from the transportation sector will continue this rising demand for materials.
In the 10 years since manufacturers deployed the first modern BEVs, the capacity of battery packs in passenger vehicles has increased while costs have decreased. Battery pack capacity in the first Nissan Leaf, released in 2010, was 24 kWh; the 2020 Tesla Model 3 has up to 75 kWh in capacity. With improved chemistries and larger energy capacities, the range of a passenger BEV has reached 400 miles on a single charge (Baldwin 2020). Meanwhile, between 2010 and 2020, the average price of battery packs decreased from $1,200 per kWh to $137 per kWh (Boudway 2020).
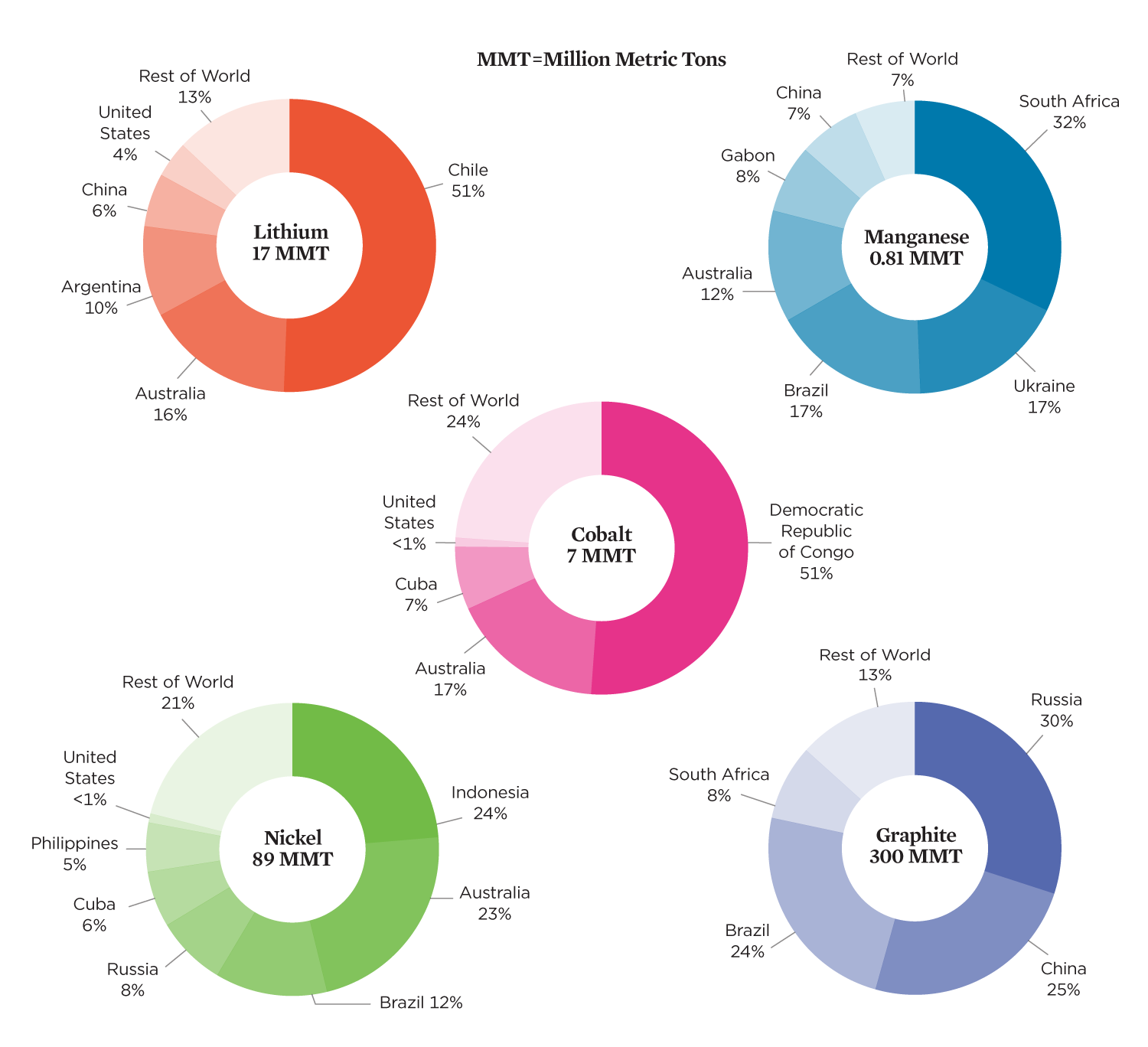
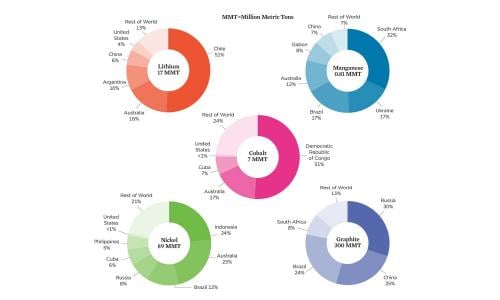
While deposits of minerals used in lithium-ion batteries are distributed widely around the world, a few countries account for most of the known “reserves”—deposits that are technically, economically, and legally feasible to extract (Figure 2, p. 4). In 2019, the global production of cobalt, nickel, and manganese each exceeded 2 percent of today’s total reserves (e.g., a 50-year supply for current reserves and demand) and of lithium and graphite, less than 1 percent. As demand has grown, so have reserves with improved extraction methods and the discovery of new mineral deposits. Reserves that are more difficult to extract, however, could exacerbate the negative impacts of mining.
Extractive industries have earned a reputation for frequently violating human rights and degrading the environment. Cobalt mining in the Democratic Republic of Congo, a country with 70 percent of the world’s existing cobalt production and more than 50 percent of cobalt reserves, has well-documented negative impacts on the environment, community health, and human rights (NMIS, n.d.a; Amnesty International 2016). Public attention to issues surrounding cobalt mining has led to commitments by several automakers and battery suppliers to improve conditions through supply chain sourcing, although much more needs to be done (WEF 2020).
Demand for Battery Materials and the Role of Recycling
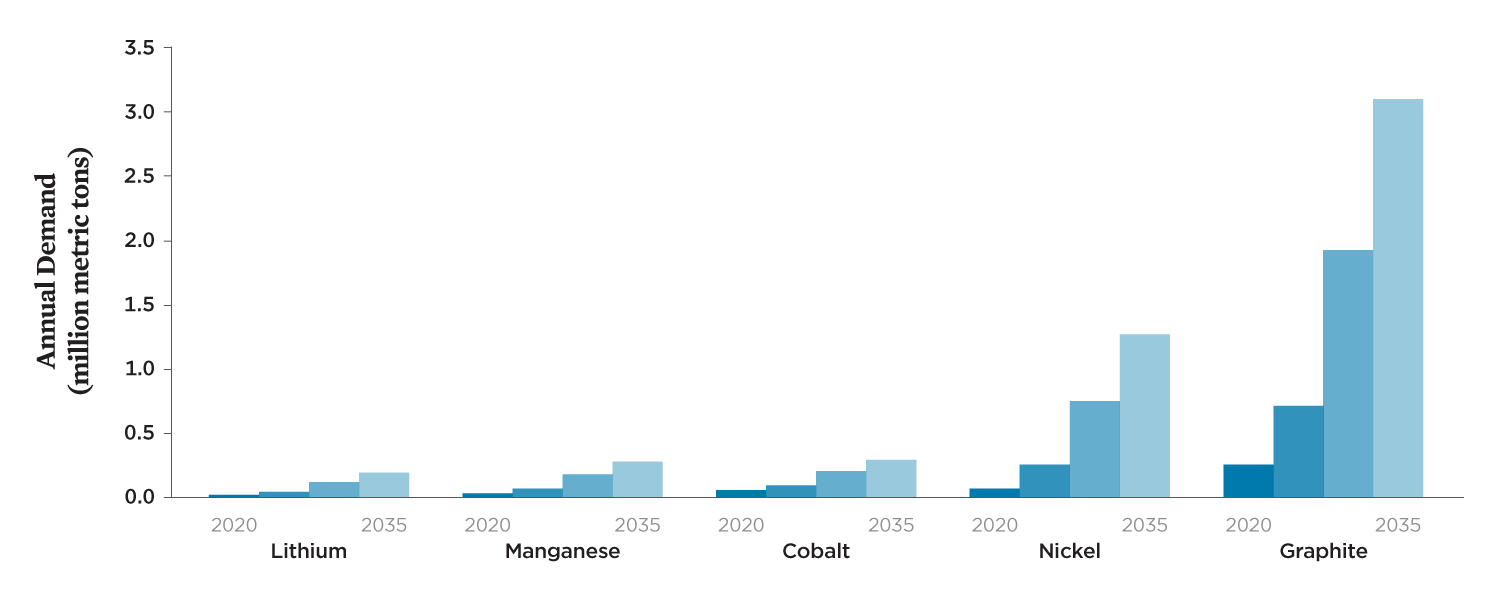
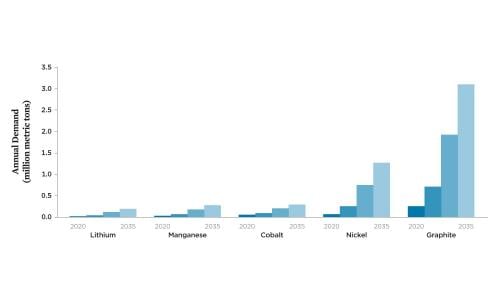
The electrification of cars and trucks will need to accelerate to avoid the most severe impacts of global warming. By one estimate, the number of passenger BEVs on US roads could increase from roughly 1 million today to 43 million by 2035 and globally from 5 million to 245 million (BNEF 2019).3 Such growth will significantly increase demand for the minerals used in batteries. Accounting for projected changes in battery chemistry, global production of lithium, nickel, manganese, cobalt, and graphite for lithium-ion batteries across all end uses could increase five- to seventeen-fold over the next 20 years, depending on the material (Figure 3, p. 5). This could strain the availability of these materials at today’s levels of economically recoverable resources and manufacturing capacity.
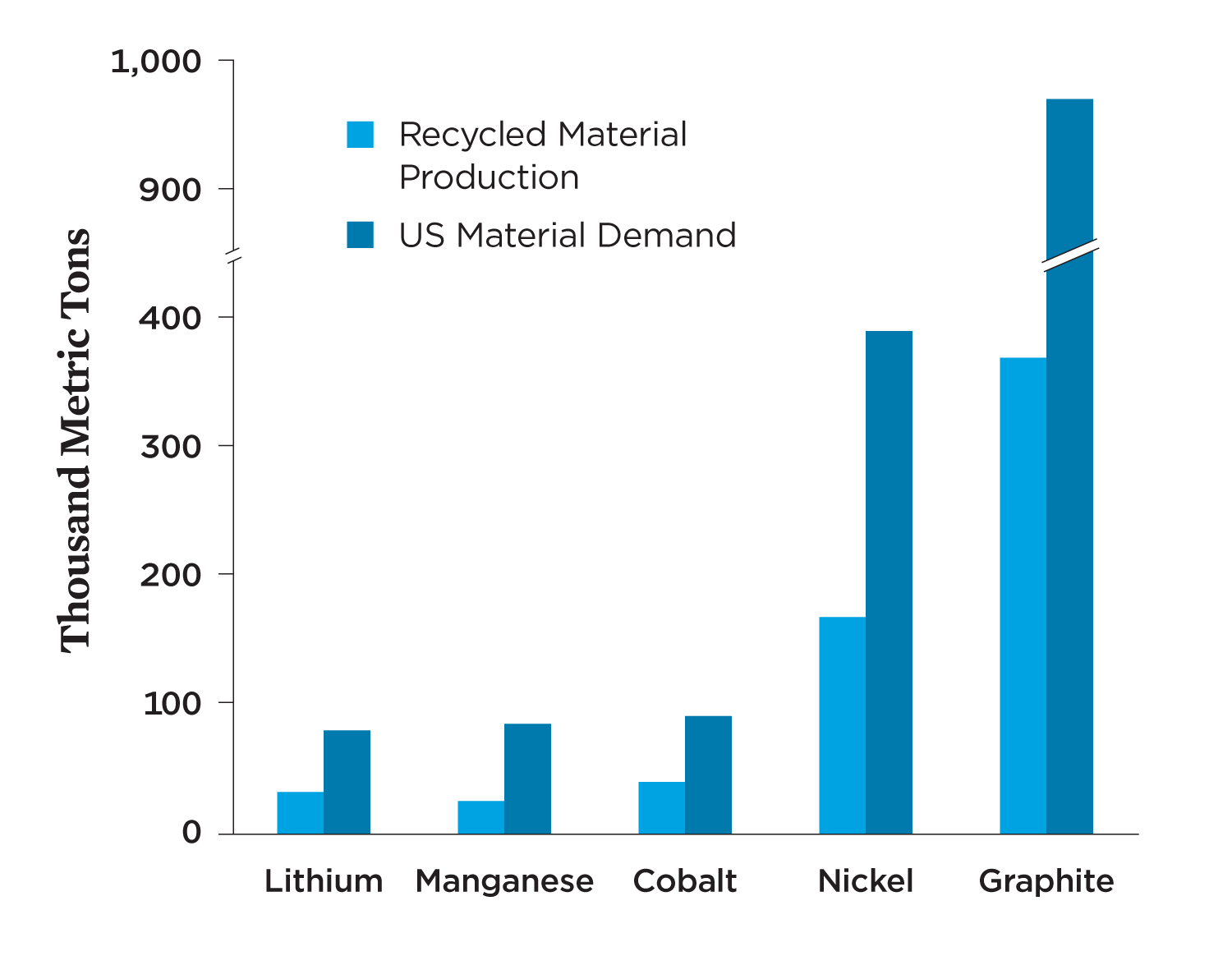
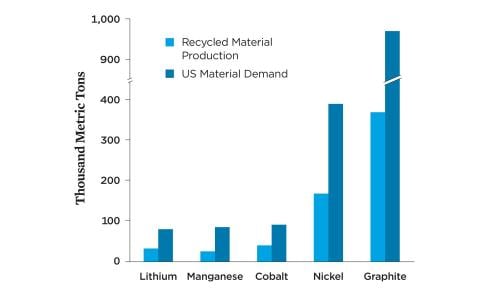
While producing many more BEVs will require new raw materials in the near term, recycled materials from used batteries could meet a significant portion of new demand in the future. Widespread battery recycling can create a more stable domestic source of materials for battery production, reduce the demand for raw materials, and minimize the risks of geopolitical disruptions of the supply chain. Assuming 95 percent collection and recovery of the relevant metals as an upper bound, as well as a shift toward low-cobalt and no-cobalt chemistries, the United States could meet about 30 to 40 percent of the anticipated material demand for lithium, nickel, manganese, cobalt, and graphite in passenger BEVs with recycled battery materials by 2035 (Figure 4, p. 6).4
The Role of Batteries in Electric Vehicle Emissions
For conventional vehicles, their operation represents their largest contribution to global warming emissions. Roughly 90 percent of global warming emissions from combustion vehicles occur at the tailpipe. In contrast, all global warming emissions associated with BEVs occur “upstream.” That is, they come from manufacturing vehicles and generating electricity to power them.
Work by the Union of Concerned Scientists and others has found that, based on today’s average sources of electricity in the United States, the total global warming emissions associated with BEVs are about 55 percent lower than those of gasoline vehicles. In parts of the country with higher levels of renewable energy—California, for example—BEVs reduce emissions by more than 70 percent. And the emissions associated with BEVs will continue to decline as the nation derives more of its electricity from renewable resources (Reichmuth 2020; Ambrose et al. 2020; Needell et al. 2016).
Upstream emissions from extracting and refining fuels and raw materials are also important considerations. For example, producing and processing crude oil into gasoline contributes an average of 24 percent of the fuel’s overall life cycle global warming emissions in the United States, depending on the source of the oil (Gordon et al. 2015; Cooney et al. 2017).
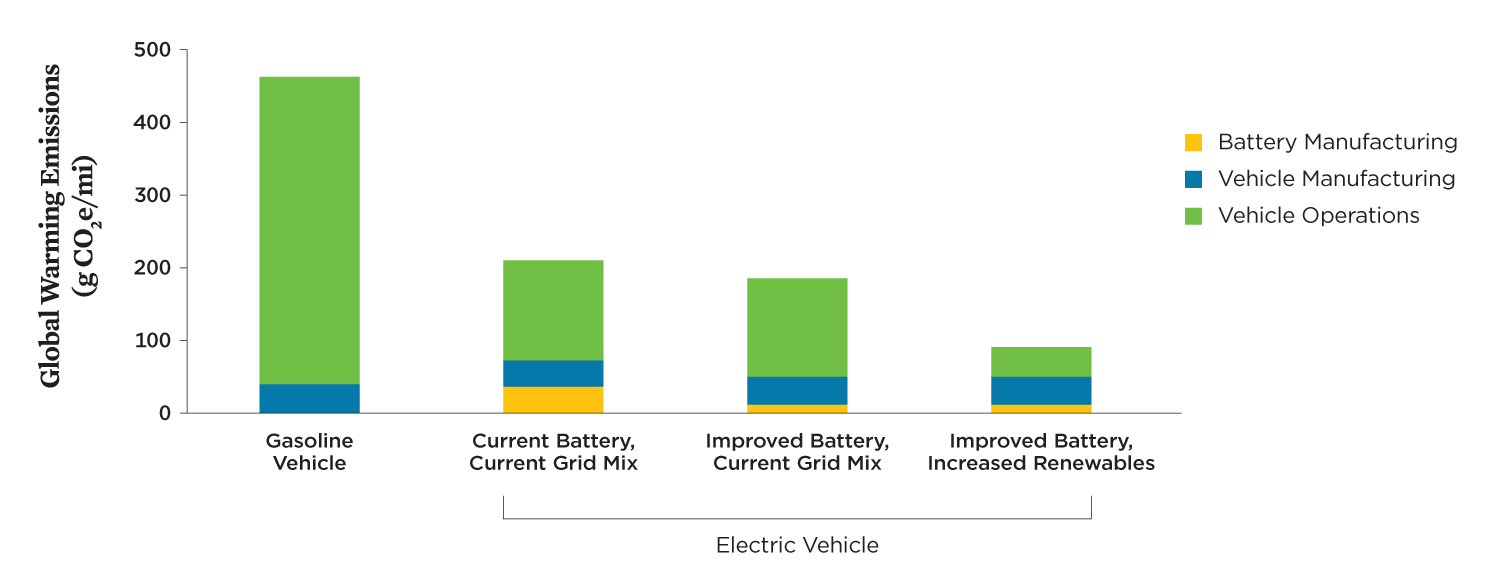
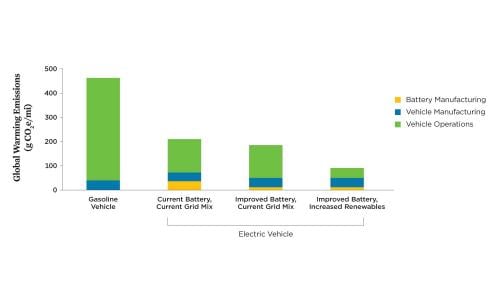
For BEVs, battery and vehicle manufacturing can con-tribute 14 percent to 24 percent of a BEV’s life cycle global warming emissions depending on where and how batteries are manufactured, as well as on the sources of raw materials (Ambrose et al. 2020). Overall, manufacturing a BEV contributes about 70 grams of carbon dioxide–equivalent emissions per mile (g CO2e/mile) compared with 40 grams for a comparably sized gasoline vehicle. But because a BEV’s operating-related emissions (i.e., vehicle charging) are relatively low, he total global warming emissions for BEVs on the average grid in the United States are less than half those for gasoline vehicles (200 g CO2e/mile vs. 450 g CO2e/mile).
Increasing the efficiency of battery manufacturing while also increasing the share of renewable energy used in assembling battery cells could reduce the global warming emissions associated with battery manufacturing by more than 40 percent (Figure 5, p. 7). Because recycling batteries reduces the need for extracting, refining, and transporting new minerals, it reduces not only emissions but also other impacts associated with these processes. Increasing the amount of renewable energy used to charge an electric vehicle, however, results in the most significant reductions in global warming emissions over the life cycle of an EV.
Reusing and Recycling Batteries
When an electric vehicle comes off the road, whether due to its age or an accident, its battery must be processed in some way. Potential end-of-life pathways include reusing the battery in other applications (“second life”), recycling the battery’s materials, and disposal. Even if a battery is reused, eventually its materials need to be recycled or disposed of. Most interest in battery recycling focuses on the cathode, which contains the highest-value materials (Box 2, p. 8). Understanding the opportunities for and barriers to recycling is critical to reducing the amount of mining needed for battery materials.
When an EV battery pack reaches the end of its useful life in a vehicle, it is still likely to retain more than two-thirds of its initial energy storage capacity—for example, the range of a BEV decreasing from 300 to 200 miles (Hossain et al. 2019). In some cases, such batteries could be refurbished for use in another vehicle or in a lower-power, stationary application. For example, a market could emerge for using second-life batteries for low-cost energy storage for utilities and electricity consumers (Mobility House 2018; Mobility House 2016). With the growing use of BEVs, the economic potential for reusing their batteries could further decrease the cost of new EVs and increase the value of used EVs.
Globally, fewer than a dozen facilities recycle EV batteries today, with a combined material processing capacity of less than 100,000 metric tons annually. For 50 kWh batteries with a gravimetric energy density of 150 watt-hours per kilogram, this recycling capacity corresponds to 300,000 EV batteries per year, or roughly 10 percent of global annual EV sales today, but 1 percent of expected annual sales in the early 2030s (BNEF 2019). In the United States, such facilities are especially limited in both number and processing capacity. One key to enabling greater recycling capacity in the United States will be increasing the domestic manufacturing of batteries.
Public Policy for Responsible Battery Management
Public policy will play an important role in enabling the wide-spread reuse of EV batteries and promoting the recycling of their constituent materials. Currently, national and regional policies for waste management and recycling do not consider the impact of large flows of EV batteries primarily because the BEV market did not exist when such policies went into place.
Lessons learned from recycling policies targeting consumer electronics and other automotive components can inform material handling and recycling policies for EV batteries. In addition, the United States can draw on the experience of other countries with major BEV markets, some of which are beginning to consider policies to address these issues. For example, China recently enacted extensive policy and guidelines for recycling EV batteries and promoting second-life uses (MIIT 2018). The policy directs manufacturers to design batteries that enable easier recycling and to provide technical information on proper storage and management. China also places responsibility for recycling on the vehicle manufacturer, a mechanism known as “extended producer responsibility.” The European Commission recently proposed extensive measures that would require collection of used batteries and set standards for recycled content in new batteries (EC 2020).
On a global level, the World Economic Forum has organized corporations, governments, and public interest groups around the world with the aim of solving key data transparency challenges related to EV batteries (WEF, n.d.). This consortium is developing standards for labeling batteries and sharing data, with the goal of providing access to critical information about battery chemistry and condition. Such information, mostly unavailable today, is critical for second-life and recycling applications and would enable the tracing of batteries’ proper-ties (e.g., chemical make-up, capacity, cycle/charging history) through the chain of ownership.
As the transition to a low-carbon, electric transportation system continues, battery recycling and reuse will become an increasingly important strategy for mitigating the potential adverse impacts of producing raw materials, disposing of waste, and securing more reliable, less damaging sources of battery materials. Such a strategy should aim at securing several essential outcomes:
- Ensuring a resilient supply of future battery materials and avoiding the need for additional development of new raw material resources;
- Planning the infrastructure needed to recycle and repurpose batteries;
- Sharing information to ensure that owners, reusers, and recyclers can access important information about battery systems;
- Encouraging battery reuse and responsible logistics to maximize the useful life of batteries and ensure the safe handling of used batteries; and
- Providing incentives and establishing requirements for sustainable practices “from mine to wheel.”
Several state, national, and global policy actions would provide the necessary framework to achieve greater reuse and recycling of battery materials:
- Set content targets for incorporating recycled materials into new battery cells as a strategy for closing the loop on the EV battery life cycle and increasing recycling.
- Set guidelines for facility permitting and land use to enable the safe transportation, storage, and recycling of used batteries.
- Set standards to label batteries with their cell chemistry and provide access to battery cycle and history data.
- Develop a waste designation for EV batteries that enables collection, responsible third-party reuse, and recycling for material recovery.
- Adopt and enforce international environmental and labor standards for mining and material processing and utilize independent third-party auditors to oversee mandatory compliance for battery suppliers.
With the growth of the BEV market, it is time for federal and state governments and international bodies to set requirements for the collection of used batteries, revise policies governing the classification and transport of used batteries, and set standards to ensure that batteries are recycled using safe and responsible methods. As the number of BEVs increases, so too will opportunities to make batteries from recycled materials. Realizing this future demands action today as we move forward on reducing transportation pollution and solving the climate crisis.
ACKNOWLEDGMENTS
This analysis was made possible by the generous support of the Hitz Foundation and UCS members. For their review of the analysis, the authors thank Jarod Kelly of Argonne National Laboratory and Ted Lamm and Ethan Elkind of the Center for Law, Energy, and the Environment at the University of California–Berkeley. The opinions expressed herein do not necessarily reflect those of the organizations that funded the work or the individuals who reviewed it. The Union of Concerned Scientists bears sole responsibility for the brief ’s content.
This is a condensed, online version of the report. Access to all figures and full report are available through download of the PDF.
Downloads
Citation
Ambrose, Hanjiro and Jimmy O’Dea. 2021. Electric Vehicle Batteries: Addressing Questions about Critical Materials and Recycling. Cambridge, MA: Union of Concerned Scientists. https://www.ucsusa.org/resources/ev-battery-recycling